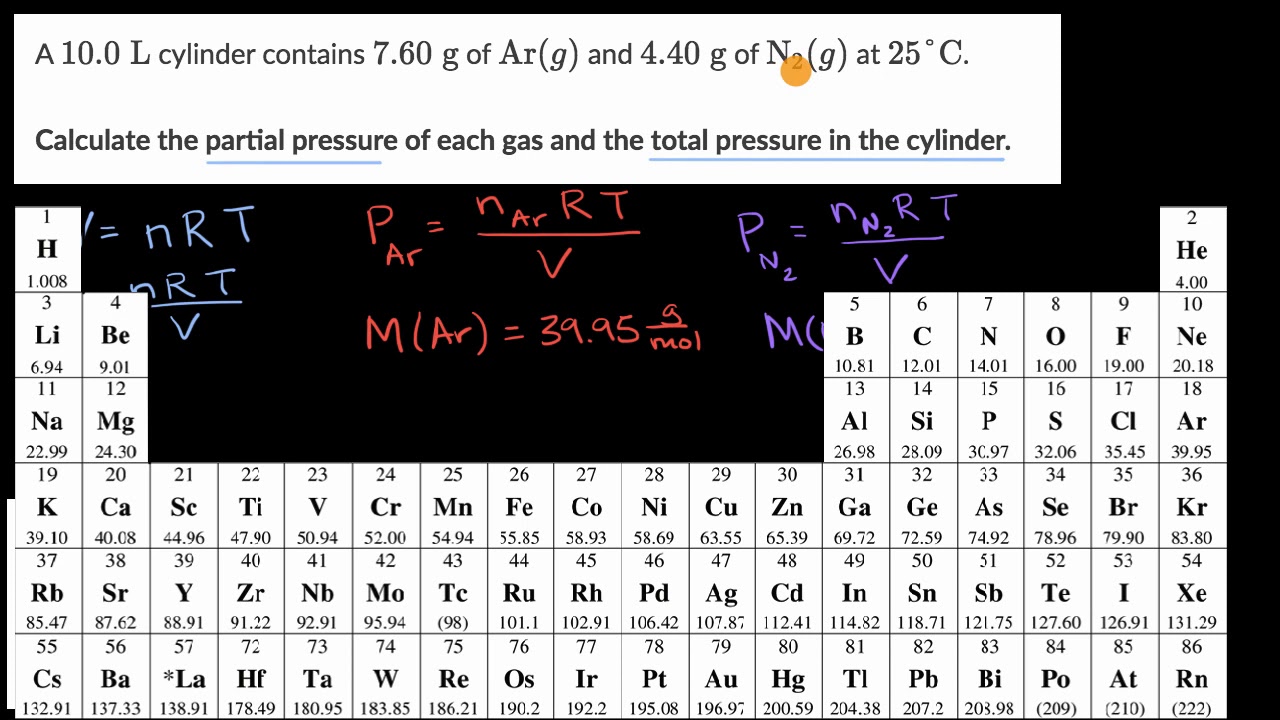
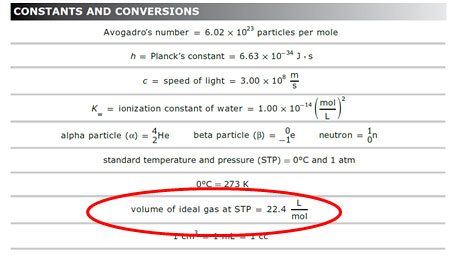
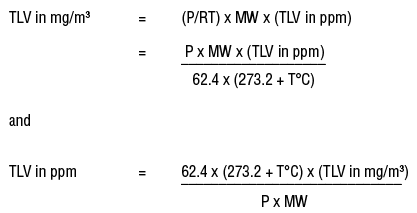
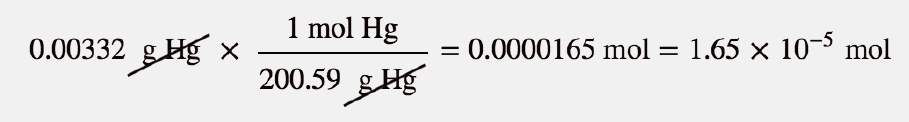Calculate w and DeltaU for the conversion of 1 mol of water at 100^(@)C to steam at 1 atm pressure.Heat of vaporisation of water at 100^(@)C is 40.670 kJ mol^(-1).Assume ideal gas
How to calculate the number of molecules of oxygen gas that occupies a volume of 224 ml at 273k and 3 atm - Quora

Ideal Gas Law Calculator (Pressure–Volume–Temperature–Amount) • Thermodynamics — Heat • Online Unit Converters
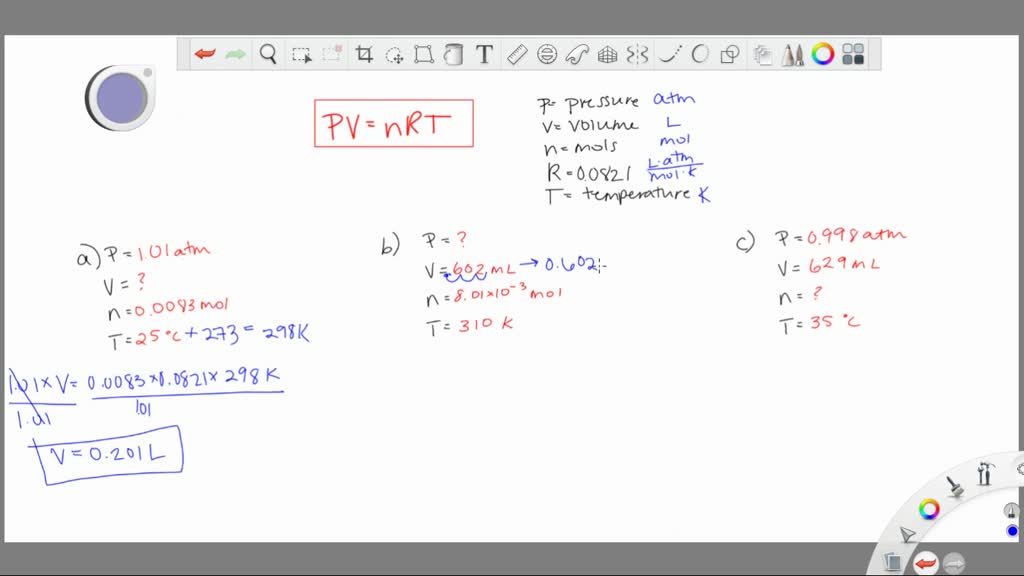
SOLVED:Given each of the following sets of values for an ideal gas, calculate the unknown quantity. a. P=1.01 \mathrm{atm} ; V=? ; n=0.00831 \mathrm{mol} T=25^{\circ} \mathrm{C} b. P=? \mathrm{atm} ; V=602 \mathrm{mL}
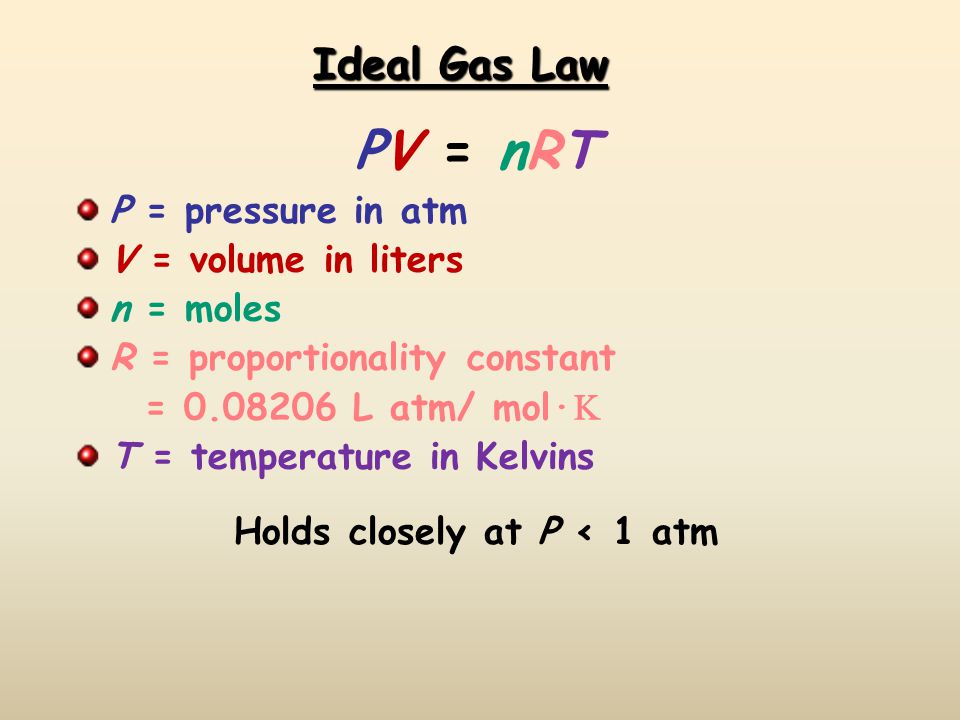
If I have 21 moles of gas held at a pressure of 3800 torr and a temperature of 627°C what is the volume of the gas? | Socratic